43 6.3 Winds and Climate
In the previous section we learned that rising air creates low pressure systems, and sinking air creates high pressure. In addition to their role in creating the surface winds, these high and low pressure systems also influence other climatic phenomena. Along the equator air is rising as it is warmed by solar radiation (section 6.2). Warm air contains more water vapor than cold air, which is why we experience humidity during the summer and not during the winter. The water content of air roughly doubles with every 10o C increase in temperature. So the air rising at the equator is warm and full of water vapor; as it rises into the upper atmosphere it cools, and the cool air can no longer hold as much water vapor, so the water condenses and forms rain. Therefore, low pressure systems are associated with precipitation, and we see wet habitats like tropical rainforests near the equator (Figure 6.3.1).
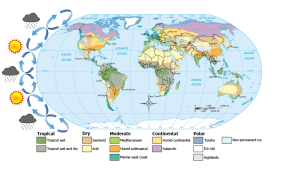
After rising and producing rain near the equator, the air masses move towards 30o latitude and sink back towards Earth as part of the Hadley convection cells. This air has lost most of its moisture after producing the equatorial rains, so the sinking air is dry, resulting in arid climates near 30o latitude in both hemispheres. Many of the major desert regions on Earth are located near 30o latitude, including much of Australia, the Middle East, and the Sahara Desert of Africa (Figure 6.3.1). The air also becomes compressed and heats up as it sinks, absorbing any moisture from the clouds and creating clear skies. Thus high pressure systems are associated with dry weather and clear skies. This cycle of high and low pressure regions continues with the Ferrel and Polar convection cells, leading to rain and the boreal forests at 60o latitude in the Northern Hemisphere (there are no corresponding large land masses at these latitudes in the Southern Hemisphere). At the poles, descending, dry air produces little precipitation, leading to the polar desert climate.
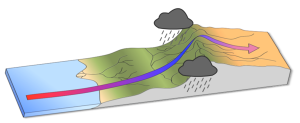
The elevation of the land also plays a role in precipitation and climactic characteristics. As moist air moves over land and encounters mountains it rises, expands, and cools because of the declining pressure and temperature. The cool air holds less water vapor, so condensation occurs and rain falls on the windward side of the mountains. As the air passes over the mountains to the leeward side, it is now dry air, and as it sinks the pressure increases, it heats back up, any moisture revaporizes, and it creates dry, deserts regions behind the mountains (Figure 6.3.2). This phenomenon is referred to as a rain shadow, and can be found in areas such as the Tibetan Plateau and Gobi Desert behind the Himalayas, Death Valley behind the Sierra Nevada mountains, and the dry San Joaquin Valley in California.
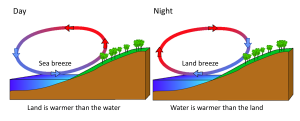
Rising and falling air are also responsible for more localized, short-term wind patterns in coastal areas. Due to the high heat capacity of water, land heats up and cools down about five times faster than water. During the day the sun heats up the land faster than it heats the water, setting up a convection cell of warmer rising air over the land and sinking cooler air over the water. This creates winds blowing from the water towards the land during the day and early evening; a sea breeze (Figure 6.3.3). The opposite occurs at night, when the land cools more quickly than the ocean. Now the ocean is warmer than the land, so air rises over the water and sinks over the land, creating a convection cell where winds blow from land towards the water. This is a land breeze, which blows at night and into the early morning (Figure 6.3.3).
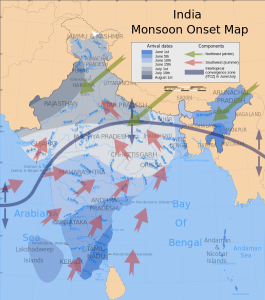
The same phenomenon leads to seasonal climatic changes in many areas. During the winter the lower pressure is over the warmer ocean, and the high pressure is over the colder land, so winds blow from land to sea. In summer the land is warmer than the ocean, causing low pressure over the land and winds to blow from the ocean towards the land. The winds blowing from the ocean contain a lot of water vapor, and as the moist air passes over land and rises, it cools and condenses causing seasonal rains, such as the summer monsoons of southeast Asia (Figure 6.3.4).
The primary surface current along the east coast of the United States is the Gulf Stream, which was first mapped by Benjamin Franklin in the 18th century (Figure 7.2.1). As a strong, fast current, it reduced the sailing time for ships traveling from the United States back to Europe, so sailors would use thermometers to locate its warm water and stay within the current.
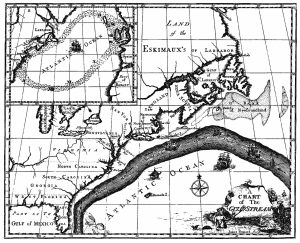
The Gulf Stream is formed from the convergence of the North Atlantic Equatorial Current bringing tropical water from the east, and the Florida Current that brings warm water from the Gulf of Mexico. The Gulf Stream takes this warm water and transports it northwards along the U.S. east coast (Figure 7.2.2). As a western boundary current, the Gulf Stream experiences western intensification (section 7.4), making the current narrow (50-100 km wide), deep (to depths of 1.5 km) and fast. With an average speed of 6.4 km/hr, and a maximum speed of about 9 km/hr, it is the fastest current in the world ocean. It also transports huge amounts of water, more than 100 times greater than the combined flow of all of the rivers on Earth.

As the Gulf Stream approaches Canada, the current becomes wider and slower as the flow dissipates and it encounters the cold Labrador Current moving in from the north. At this point, the current begins to meander, or change from a fast, straight flow to a slower, looping current (Figure 7.2.2). Often these meanders loop so much that they pinch off and form large rotating water masses called rings or eddies, that separate from the Gulf Stream. If an eddy pinches off from the north side of the Gulf Stream, it entraps a mass of warm water and moves it north into the surrounding cold water of the North Atlantic. These warm core rings are shallow, bowl-shaped water masses about 1 km deep, and about 100 km across, that rotate clockwise as they carry warm water in to the North Atlantic (Figure 7.2.3). If the meanders pinch off at the southern boundary of the Gulf Stream, they form cold core rings that rotate counterclockwise and move to the south. Cold core rings are cone-shaped water masses extending down to over 3.5 km deep, and may be over 500 km wide at the surface.

After the Gulf Stream meets the cold Labrador Current, it joins the North Atlantic Current, which transports the warm water towards Europe, where it moderates the European climate. It is estimated that Northern Europe is up to 9o C warmer than expected because of the Gulf Stream, and the warm water helps to keep many northern European ports ice-free in the winter.
In the east, the Gulf Stream merges into the Sargasso Sea, which is the area of the ocean within the rotation center of the North Atlantic gyre. The Sargasso Sea gets its name from the large floating mats of the marine algae Sargassum that are abundant on the surface (Figure 7.2.4). These Sargassum mats may play an important role in the early life stages of sea turtles, who may live and feed within the algae for many years before reaching adulthood.

If one thing has been constant about Earth’s climate over geological time, it is its constant change. In the geological record, we can see this in the evidence of glaciations in the distant past, and we can also detect periods of extreme warmth by looking at the isotope composition of seafloor sediments. Not only has the climate changed frequently, the temperature fluctuations have been very significant. Today’s mean global temperature is about 15°C. However, during its coldest periods, the global mean was as cold as -50°C, while at various times during the Paleozoic and Mesozoic and during the Paleocene-Eocene thermal maximum, it was close to 30°C.
There are two parts to climate change, the first one is known as climate forcing, which is when conditions change to give the climate a little nudge in one direction or the other. The second part of climate change, and the one that typically does most of the work, is what we call a feedback. When a climate forcing changes the climate a little, a whole series of environmental changes take place, many of which either exaggerate the initial change (positive feedback), or suppress the change (negative feedback).
An example of a climate forcing mechanism is the increase in the amount of carbon dioxide (CO2) in the atmosphere that results from our use of fossil fuels. CO2 traps heat in the atmosphere and leads to climate warming. Warming changes vegetation patterns; contributes to the melting of snow, ice, and permafrost; causes sea level to rise; reduces the solubility of CO2 in sea water; and has a number of other minor effects. Most of these changes contribute to more warming. Melting of permafrost, for example, is a strong positive feedback because frozen soil contains trapped organic matter that is converted to CO2 and methane (CH4) when the soil thaws. Both these gases accumulate in the atmosphere and add to the warming effect. On the other hand, if warming causes more vegetation growth, that vegetation should absorb CO2, thus reducing the warming effect, which would be a negative feedback. Under our current conditions — a planet that still has lots of glacial ice and permafrost — most of the feedbacks that result from a warming climate are positive feedbacks and so the climate changes that we cause get naturally amplified by natural processes.
Natural Climate Forcing
Natural climate forcing has been going on throughout geological time. A wide range of processes has been operating at widely different time scales, from a few years to billions of years. The longest-term natural forcing variation is related to the evolution of the Sun. Like most other stars of a similar mass, our Sun is evolving. For the past 4.6 billion years, its rate of nuclear fusion has been increasing, and it is now emitting about 40% more energy (as light) than it did at the beginning of geological time. A difference of 40% is big, so it’s a little surprising that the temperature on Earth has remained at a reasonable and habitable temperature for all of this time. The mechanism for that relative climate stability has been the evolution of our atmosphere from one that was dominated by CO2, and also had significant levels of CH4 — both greenhouse gasses — to one with only a few hundred parts per million of CO2 and just under 1 part per million of CH4. Those changes to our atmosphere have been no accident; over geological time, life and its metabolic processes have evolved (such as the evolution of photosynthetic bacteria that consume CO2) and changed the atmosphere to conditions that remained cool enough to be habitable.
The position of the Earth relative to the Sun is another important component of natural climate forcing. Earth’s orbit around the Sun is nearly circular, but like all physical systems, it has natural oscillations. First, the shape of the orbit changes on a regular time scale (close to 100,000 years) from being close to circular to being very slightly elliptical. But the circularity of the orbit is not what matters; it is the fact that as the orbit becomes more elliptical, the position of the Sun within that ellipse becomes less central or more eccentric (Figure 6.5.1a). Eccentricity is important because when it is high, the Earth-Sun distance varies more from season to season than it does when eccentricity is low.
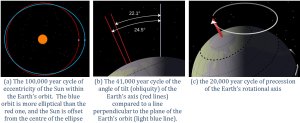
Second, Earth rotates around an axis through the North and South Poles, and that axis is at an angle to the plane of Earth’s orbit around the Sun (Figure 6.5.1b). The angle of tilt (also known as obliquity) varies on a time scale of 41,000 years. When the angle is at its maximum (24.5°), Earth’s seasonal differences are accentuated. When the angle is at its minimum (22.1°), seasonal differences are minimized. The current hypothesis is that glaciation is favored at low seasonal differences as summers would be cooler and snow would be less likely to melt and more likely to accumulate from year to year. Third, the direction in which Earth’s rotational axis points also varies, on a time scale of about 20,000 years (Figure 6.5.1c). This variation, known as precession, means that although the North Pole is presently pointing to the star Polaris (the pole star), in 10,000 years it will point to the star Vega. The importance of eccentricity, tilt, and precession to Earth’s climate cycles (now known as Milankovitch Cycles) was first pointed out by Yugoslavian engineer and mathematician Milutin Milankovitch in the early 1900s. Milankovitch recognized that although the variations in the orbital cycles did not affect the total amount of insolation (light energy from the Sun) that Earth received, it did affect where on Earth that energy was strongest.
Volcanic eruptions don’t just involve lava flows and exploding rock fragments; various particulates and gases are also released, the important ones being sulphur dioxide and CO2. Sulphur dioxide is an aerosol that reflects incoming solar radiation and has a net cooling effect that is short lived (a few years in most cases, as the particulates settle out of the atmosphere within a couple of years), and doesn’t typically contribute to longer-term climate change. Volcanic CO2 emissions can contribute to climate warming but only if a greater-than-average level of volcanism is sustained over a long time (at least tens of thousands of years). It is widely believed that the catastrophic end-Permian extinction (at 250 Ma) resulted from warming initiated by the eruption of the massive Siberian Traps over a period of at least a million years.
Ocean currents are important to climate, and currents also have a tendency to oscillate. Glacial ice cores show clear evidence of changes in the Gulf Stream that affected global climate on a time scale of about 1,500 years during the last glaciation. The east-west changes in sea-surface temperature and surface pressure in the equatorial Pacific Ocean, known as the El Niño Southern Oscillation or ENSO varies on a much shorter time scale of between two and seven years. These variations tend to garner the attention of the public because they have significant climate implications in many parts of the world. The strongest El Niños in recent decades were in 1983, 1998, and 2015 and those were very warm years from a global perspective. During a strong El Niño, the equatorial Pacific sea-surface temperatures are warmer than normal and heat the atmosphere above the ocean, which leads to warmer-than-average global temperatures.
Climate Feedbacks
As already stated, climate feedbacks are critically important in amplifying weak climate forcings into full-blown climate changes. Since Earth still has a very large volume of ice, mostly in the continental ice sheets of Antarctica and Greenland, but also in alpine glaciers and permafrost, melting is one of the key feedback mechanisms. Melting of ice and snow leads to several different types of feedbacks, an important one being a change in albedo, or the reflectivity of a surface. Earth’s various surfaces have widely differing albedos, expressed as the percentage of light that reflects off a given material. This is important because most solar energy that hits a very reflective surface is not absorbed and therefore does little to warm Earth. Water in the oceans or on a lake is one of the darkest surfaces, reflecting less than 10% of the incident light, while clouds and snow or ice are among the brightest surfaces, reflecting 70% to 90% of the incident light. When sea ice melts, as it has done in the Arctic Ocean at a disturbing rate over the past decade, the albedo of the area affected changes dramatically, from around 80% down to less than 10%. Much more solar energy is absorbed by the water than by the pre-existing ice, and the temperature increase is amplified. The same applies to ice and snow on land, but the difference in albedo is not as great. When ice and snow on land melt, sea level rises. (Sea level is also rising because the oceans are warming and that increases their volume). A higher sea level means a larger proportion of the planet is covered with water, and since water has a lower albedo than land, more heat is absorbed and the temperature goes up a little more. Since the last glaciation, sea-level rise has been about 125 m; a huge area that used to be land is now flooded by heat-absorbent seawater. During the current period of anthropogenic climate change, sea level has risen only about 20 cm, and although that doesn’t make a big change to albedo, sea-level rise is accelerating.
Most of northern Canada, Alaska, Russia, and Scandinavia has a layer of permafrost that ranges from a few centimeters to hundreds of meters in thickness. Permafrost is a mixture of soil and ice and it also contains a significant amount of trapped organic carbon that is released as CO2 and CH4 when the permafrost breaks down. Because the amount of carbon stored in permafrost is in the same order of magnitude as the amount released by burning fossil fuels, this is a feedback mechanism that has the potential to equal or surpass the forcing that has unleashed it. In some polar regions, including northern Canada, permafrost includes methane hydrate, a highly concentrated form of CH4 trapped in solid form. Breakdown of permafrost releases this CH4. Even larger reserves of methane hydrate exist on the seafloor, and while it would take significant warming of ocean water down to a depth of hundreds of meters, this too is likely to happen in the future if we don’t limit our impact on the climate. There is strong isotopic evidence that the Paleocene-Eocene thermal maximum was caused, at least in part, by a massive release of sea-floor methane hydrate.
There is about 45 times as much carbon in the ocean (as dissolved bicarbonate ions, HCO3-) as there is in the atmosphere (as CO2), and there is a steady exchange of carbon between the two reservoirs (see section 5.5). But the solubility of CO2 in water decreases as the temperature goes up. In other words, the warmer it gets, the more oceanic bicarbonate that gets transferred to the atmosphere as CO2. That makes CO2 solubility another positive feedback mechanism. Vegetation growth responds positively to both increased temperatures and elevated CO2 levels, and so in general, it represents a negative feedback to climate change because the more the vegetation grows, the more CO2 is taken from the atmosphere. But it’s not quite that simple, because when trees grow bigger and more vigorously, forests become darker (they have lower albedo) so they absorb more heat. Furthermore, climate warming isn’t necessarily good for vegetation growth; some areas have become too hot, too dry, or even too wet to support the plant community that was growing there, and it might take centuries for something to replace it successfully. All of these positive (and negative) feedbacks work both ways. For example, during climate cooling, growth of glaciers leads to higher albedos, and formation of permafrost results in storage of carbon that would otherwise have returned quickly to the atmosphere.
Anthropogenic Climate Change
When we talk about anthropogenic climate change, we are generally thinking of the industrial era, which really got going when we started using fossil fuels (coal to begin with, and later oil and natural gas) to drive machinery and trains, and to generate electricity. That was around the middle of the 18th century. The issue with fossil fuels is that they involve burning carbon that was naturally stored in the crust over hundreds of millions of years as part of Earth’s process of counteracting the warming Sun.
A rapidly rising population, the escalating level of industrialization and mechanization of our lives, and an increasing dependence on fossil fuels have driven the anthropogenic climate change of the past century. The trend of mean global temperatures since 1850 is shown in Figure 6.5.2. For approximately the past 55 years, the temperature has increased at a relatively steady and disturbingly rapid rate, especially compared to past changes. The average temperature now is approximately 1.1°C higher than before industrialization, and two-thirds of this warming has occurred since 1975.
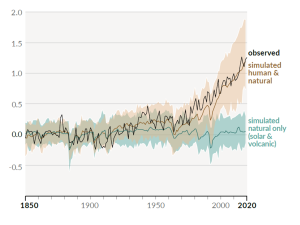
The Intergovernmental Panel on Climate Change (IPCC), established by the United Nations in 1988, is responsible for reviewing the scientific literature on climate change and issuing periodic reports on several topics, including the scientific basis for understanding climate change, our vulnerability to observed and predicted climate changes, and what we can do to limit climate change and minimize its impacts. Figure 6.5.3, from the sixth report of the IPCC, issued in preliminary form in 2021, shows the relative contributions of various greenhouse gases and other factors to current climate forcing, based on the changes from levels that existed in 1750.
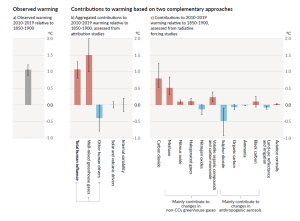
The biggest anthropogenic contributor to warming is the emission of CO2, which accounts for 50% of positive forcing. CH4 and its atmospheric derivatives (CO2, H2O, and O3) account for 29%, and the halocarbon gases (mostly leaked from air-conditioning appliances) and nitrous oxide (N2O) (from burning fossils fuels) account for 5% each. Carbon monoxide (CO) (also produced by burning fossil fuels) accounts for 7%, and the volatile organic compounds other than methane (NMVOC) account for 3%. CO2 emissions come mostly from coal- and gas-fired power stations, motorized vehicles (cars, trucks, and aircraft), and industrial operations (e.g., smelting), and indirectly from forestry. CH4 emissions come from production of fossil fuels (escape from coal mining and from gas and oil production), livestock farming (mostly beef), landfills, and wetland rice farming. N2O and CO come mostly from the combustion of fossil fuels. In summary, close to 70% of our current greenhouse gas emissions come from fossil fuel production and use, while most of the rest comes from agriculture and landfills. Figure 6.5.4 shows the IPCC’s projections for temperature increases over the next 100 years as a result of these increasing greenhouse gases.

Impacts of Climate Change
We’ve all experienced the effects of climate change over the past decade. However, it’s not straightforward for climatologists to make the connection between a warming climate and specific weather events, and most are justifiably reluctant to ascribe any specific event to climate change. In this respect, the best measures of climate change are those that we can detect over several decades, such as the temperature changes shown in Figure 6.5.2, or the sea level rise shown in Figure 6.5.5. As already stated, sea level has risen approximately 20 cm since 1750, and that rise is attributed to both warming (and therefore expanding) seawater and melting glaciers and other land-based snow and ice (melting of sea ice does not contribute directly to sea level rise as it is already floating in the ocean).

Projections for sea level rise to the end of this century vary widely. This is in large part because we do not know which of the above climate change scenarios (Figure 6.5.4) we will most closely follow, but many are in the range from 0.5 m to 2.0 m. One of the problems in predicting sea level rise is that we do not have a strong understanding of how large ice sheets, such as Greenland and Antarctica, will respond to future warming. Another issue is that the oceans don’t respond immediately to warming. For example, with the current amount of warming, we are already committed to a future sea level rise of between 1.3 m and 1.9 m, even if we could stop climate change today. This is because it takes decades to centuries for the existing warming of the atmosphere to be transmitted to depth within the oceans and to exert its full impact on large glaciers. Most of that committed rise would take place over the next century, but some would be delayed longer. And for every decade that the current rates of climate change continue, that number increases by another 0.3 m. In other words, if we don’t make changes quickly, by the end of this century we’ll be locked into 3 m of future sea level rise. In a 2008 report, the Organization for Economic Co-operation and Development (OECD) estimated that by 2070 approximately 150 million people living in coastal areas could be at risk of flooding due to the combined effects of sea level rise, increased storm intensity, and land subsidence. The assets at risk (buildings, roads, bridges, ports, etc.) are in the order of $35 trillion ($35,000,000,000,000). Countries with the greatest exposure of population to flooding are China, India, Bangladesh, Vietnam, U.S.A., Japan, and Thailand. Some of the major cities at risk include Shanghai, Guangzhou, Mumbai, Kolkata, Dhaka, Ho Chi Minh City, Tokyo, Miami, and New York.
One of the other risks for coastal populations, besides sea level rise, is that climate warming is also associated with an increase in the intensity of tropical storms (e.g., hurricanes or typhoons; see section 6.4), which almost always bring serious flooding from intense rain and storm surges. Some recent examples are New Orleans in 2005 with Hurricane Katrina, and New Jersey and New York in 2012 with Hurricane Sandy. Tropical storms get their energy from the evaporation of warm seawater in tropical regions. In the Atlantic Ocean, this takes place between 8° and 20° N in the summer. Figure 6.5.6 shows the variations in the sea-surface temperature (SST) of the tropical Atlantic Ocean (in blue) versus the amount of power represented by Atlantic hurricanes between 1950 and 2008 (in red). Not only has the overall intensity of Atlantic hurricanes increased with the warming since 1975, but the correlation between hurricanes and sea-surface temperatures is very strong over that time period.

The geographical ranges of diseases and pests, especially those caused or transmitted by insects, have been shown to extend toward temperate regions because of climate change. West Nile virus and Lyme disease are two examples that already directly affect North Americans, while dengue fever could be an issue in the future (dengue became a "nationally notifiable condition" in the United States in 2010). For several weeks in July and August of 2010, a massive heat wave affected western Russia, especially the area southeast of Moscow, and scientists have stated that climate change was a contributing factor. Temperatures soared to over 40°C, as much as 12°C above normal over a wide area, and wildfires raged in many parts of the country. Over 55,000 deaths are attributed to the heat and to respiratory problems associated with the fires. A summary of the impacts of climate change on natural disasters is given in Figure 6.5.7. The major types of disasters related to climate are floods and storms, but the health implications of extreme temperatures are also becoming a great concern. In the decade 1971 to 1980, extreme temperatures were the fifth most common natural disasters; by 2001 to 2010, they were the third most common.

In the previous chapter the major wind patterns on Earth were derived. It is these prevailing winds that blow across the water surface to create the major ocean surface currents. However, only about 2% of the wind energy is actually transferred to the water, so a 50 knot wind only creates a 1 knot current. Furthermore, wind-driven surface currents only affect the top 100-200m of water, meaning surface currents only involve about 10% of the world’s ocean water. In section 7.8 we will examine deep, thermohaline circulation, which impacts around 90% of the ocean water.
Surface currents generally move in the same direction as the winds that created them. However, because of Coriolis deflection, the surface currents are offset approximately 45o relative to the wind direction; 45o to the right in the Northern Hemisphere, and 45o to the left in the Southern Hemisphere. This creates a general circulation pattern where in both hemispheres, surface currents flow east to west between the equator and 30o latitude, west to east between 30o and 60o, and east to west between 60o and the poles (Figure 7.1.1).
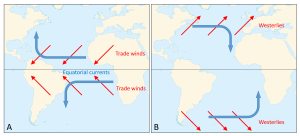
The trade winds create the equatorial currents that flow east to west along the equator; the North Equatorial and South Equatorial currents. If there were no continents, these surface currents would travel all the way around the Earth, parallel to the equator. However, the presence of the continents prevents this unimpeded flow. When these equatorial currents reach the continents, they are diverted and deflected away from the equator by the Coriolis Effect; deflection to the right in the Northern Hemisphere and to the left in the Southern Hemisphere. These currents then become western boundary currents; currents that run along the western side of the ocean basin (i.e. the east coasts of the continents). Since these currents come from the equator, they are warm water currents, bringing warm water to the higher latitudes and distributing heat throughout the ocean.
At the same time, between 30-60o latitude the westerlies move surface water towards the east. The Coriolis Effect and the presence of the continents deflect the currents towards the equator, creating eastern boundary currents (on the eastern side of the ocean basins). These currents come from high latitude areas, so they deliver cold water to the lower latitudes. Together, these currents combine to create large-scale circular patterns of surface circulation called gyres. In the Northern Hemisphere the gyres rotate to the right (clockwise), while in the Southern Hemisphere the gyres rotate to the left (counterclockwise).
There are five major gyres in the oceans; the North Atlantic, South Atlantic, North Pacific, South Pacific, and Indian (Figure 7.1.2). The North Pacific gyre is composed of the North Equatorial Current on its southern boundary, which turns into the Kuroshio Current (a.k.a. the Japan Current) bringing warm water north towards Japan. The Kuroshio flows into the North Pacific Current which moves east towards North America, where it becomes the California Current to complete the gyre. The North Atlantic gyre is formed by the North Equatorial Current flowing into the Gulf Stream along the east coast of the United States. The Gulf Stream merges into the North Atlantic Current to move water towards Europe, which then becomes the Canary Current as it moves south to join the North Equatorial Current.

Near Antarctica the circulation is somewhat different. Because there is little in the way of continental land masses between 50-60o south, the surface current created by the westerly winds can make its way completely around the Earth, creating the Antarctic Circumpolar Current (ACC) or West Wind Drift (WWD) that flows from west to east (Figure 7.1.2). The Antarctic Circumpolar Current is the only current that connects all of the major ocean basins, and in terms of the amount of water that it transports, it is the largest surface current on Earth. Above 60o latitude the prevailing winds are the polar easterlies, which create a current flowing from east to west along the edge of the Antarctic continent, the East Wind Drift or the Antarctic Coastal Current.
The Antarctic Circumpolar Current creates the southern boundary for all of the Southern Hemisphere gyres. In the South Pacific gyre the ACC becomes the Peru Current (also known as the Humboldt Current) moving up the west coast of South America, before joining the South Equatorial Current. The South Equatorial Current flows southwards as the East Australia Current, before completing the gyre with the ACC. The South Atlantic gyre is composed of the South Equatorial Current, the Brazil Current, the ACC, and the Benguela Current. Finally, the currents making up the Indian gyre are the ACC, the West Australia Current, the South Equatorial Current, and the Agulhas Current.
Not all of the equatorial water that is moved westward by the trade winds and reaches the continents gets transported to higher latitudes in the gyres, because the Coriolis Effect is weakest along the equator. Instead, some of the water piles up along the western edge of the ocean, and then flows eastward due to gravity, creating narrow Equatorial Countercurrents between the North and South Equatorial Currents (Figure 7.1.2). Some of this water also moves east as equatorial undercurrents that flow at depths between 50-200 m, underneath the Equatorial Currents. These undercurrents are called the Lomonosov Current in the Atlantic, and the Cromwell Current in the Pacific.
So how did the oceans form in the first place? The early Earth was formed through the accretion of various materials, and that a period of melting and intense volcanic activity followed. The materials that accreted on the early Earth contained the components that would eventually become our oceans and atmosphere. There are a few hypotheses concerning the origin of the oceans. One suggests that under the high pressures found in the Earth's interior, gases remain dissolved in magma. As these magmas rise to the surface through volcanic activity, the pressure is reduced and the gases are released through a process called outgassing. Volcanic activity releases many different gases, including water vapor, carbon dioxide (CO2), sulfur dioxide (SO2), carbon monoxide (CO), hydrogen sulfide (H2S), hydrogen gas, nitrogen, and methane (CH4). Lighter gases such as hydrogen and helium dissipated into space, but the heavier gases remained and formed Earth's early atmosphere, and potentially surface water. Another idea is that during this early bombardment, water was brought to Earth through comets, which are mostly dust and ice, and/or meteorites that may have contained traces of water that could have accumulated on the Earth's surface. These hypotheses are not mutually exclusive, and it's possible that all of them contributed to the formation of the oceans.
The rise of atmospheric oxygen
As the early Earth cooled, the water vapor in the atmosphere condensed and fell as rain. By about 4 billion years ago, the first permanent accumulations of water were present on Earth, forming the oceans and other bodies of water. Water moves between these different reservoirs through the hydrological cycle. Water is evaporated from the oceans, lakes, streams, the surface of the land, and plants (transpiration) by solar energy (Figure 5.2.1). It is moved through the atmosphere by winds and condenses to form clouds of water droplets or ice crystals. It comes back down as rain or snow and then flows through streams and rivers, into lakes, and eventually back to the oceans. Water on the surface and in streams and lakes infiltrates the ground to become groundwater. Groundwater slowly moves through the rock and surface materials; some returns to other streams and lakes, and some goes directly back to the oceans.
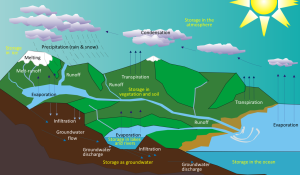
Water is stored in various reservoirs as it moves through this cycle. The largest, by far, is the oceans, accounting for 97% of the volume (Figure 5.2.2). Of course, that water is salty. The remaining 3% is fresh water. Two-thirds of our fresh water is stored in ice and one-third is stored as groundwater. The remaining fresh water — about 0.03% of the total — is stored in lakes, streams, vegetation, and the atmosphere.

To put that in perspective, let’s think about putting all of Earth’s water into a 1 L jug. We start by almost filling the jug with 970 ml of water and 34 g of salt. Then we add one regular-sized (~20 mL) ice cube (representing glacial ice) and two teaspoons (~10 mL) of groundwater. All of the water that we see around us in lakes and streams and up in the sky can be represented by adding three more drops from an eyedropper.
Although the proportion of Earth’s water that is in the atmosphere is tiny, the actual volume is huge. At any given time, there is the equivalent of approximately 13,000 km3 of water in the air in the form of water vapor and water droplets in clouds. Water is evaporated from the oceans, vegetation, and lakes at a rate of 1,580 km3 per day, and just about exactly the same volume falls as rain and snow every day, over both the oceans and land. The precipitation that falls on land goes back to the ocean in the form of stream flow (117 km3/day) and groundwater flow (6 km3/day).
How did the oceans get salty?
Outgassing was responsible for ocean formation, but how did the ocean water get salty? Most of the salts and dissolved elements in the ocean were probably outgassed along with the water vapor, so the ocean has probably always been about as salty as it is now. But we know that rainfall and other processes weather rocks on the Earth's surface, and runoff carries dissolved substances into the ocean, contributing to its salinity. Yet despite this constant input, the ocean’s salt composition remains essentially the same. Therefore, the rate of input of new material must be balanced by the rate of removal; in other words, the oceans are in a steady state in regards to salinity.
There are multiple pathways through which dissolved ions enter the ocean; runoff from streams and rivers, volcanic activity, hydrothermal vents (see section 2.11), dissolution or decay of substances in the ocean, and groundwater input. Ions are removed from seawater as they are incorporated by living organisms (for example in shell production) or sediments, sea spray, percolation of water into the crust, or when sea water gets isolated from the ocean and evaporates.
The relationship between the input and removal of an ion can be examined through the concept of residence time, which is the average length of time a single atom of an element remains in the ocean before being removed. Residence time is calculated as:
[latex]\text { Residence time} =\frac{\text{amount of the substance in the ocean}}{\text {the rate at which the substance is added or removed}}[/latex]
There is great variation in residence times for different substances (Table 5.2.1). Generally speaking, substances that are readily used in biological processes have short residence times, as they are used up as they become available. Substances with longer residence times are less reactive, and may be a part of long-scale geological cycles.
Table 5.2.1 Residence times for some constituents of sea water
| Constituent | Residence time (years) |
|---|---|
| Chloride (Cl-) | 100,000,000 |
| Sodium (Na+) | 68,000,000 |
| Calcium (Ca2+) | 1,000,000 |
| Water | 4100 |
| Iron (Fe) | 200 |
So what about lakes? They are subjected to runoff and river input, so why aren't they salty like the oceans? One reason is that compared to the oceans, lakes and ponds are relatively temporary phenomena, so they do not last long enough to accumulate the same levels of ions as the oceans. Furthermore, lakes often have rivers flowing both into and out of them, so many ions are removed through the outflow, eventually finding their way to the oceans. The oceans only receive river input; there are no rivers flowing out of the ocean to remove these materials, so they are found in greater abundance in sea water. It should be noted that there are some lakes that contain water whose salt content may rival or exceed that of the ocean; these lakes usually lack river outflow. The Great Salt Lake in the western United States is an example.
By Paul Webb, used under a CC-BY 4.0 international license. Download this book for free at https://rwu.pressbooks.pub/webboceanography/front-matter/preface/
Modified from "Physical Geology" by Steven Earle used under a CC-BY 4.0 international license. Download this book for free at http://open.bccampus.ca
Tsunamis loom large in popular culture, but there are a number of misconceptions about these large waves. First, tsunamis have nothing to do with the tides, so it is a misnomer to refer to them as "tidal waves." There are actual tidal waves (see section 3.5), but they are not related to tsunamis. Second, the giant, curling wave that is taller than skyscrapers and destroys cities in science fiction movies is also a fabrication, as tsunamis do not behave that way, as described below.
Tsunamis are large waves that are usually the result of seismic activity, such as the rising or falling of the seafloor due to earthquakes, although volcanic activity and landslides can also cause tsunamis in the form of splash waves (see section 3.1). As the seafloor rises or falls, so does the water column above it, creating waves. Only vertical seismic disturbances cause tsunamis, not horizontal movements. These vertical seafloor movements are usually less than 10 m high, so the resulting wave will be of an equal or lesser height at sea. While the tsunamis have a relatively small height at the point of origin, they have very long wavelengths (100-200 km). Because of the long wavelength, they behave as shallow water waves throughout the entire ocean; the depth of the ocean is always shallower than half of their wavelength. As shallow water waves, their speed depends on water depth, but they can still travel at speeds over 750 km/hr (Figure 3.4.1)!
When tsunamis approach land, they behave just like any other wave; as the depth becomes shallower, the waves slow down and the wave height begins to increase. However, contrary to popular belief, tsunamis do not arrive on shore as giant, cresting waves. Since their wavelength is so long, it is impossible for their height to ever exceed 1/7 of their wavelength, so the waves don’t actually curl or break. Instead, they usually hit the shore as sudden surges of water causing a very rapid increase in sea level, like that of an enormous rise in tide. It may take several minutes for the wave to pass, during which time sea level can rise to 40 m higher than usual.
Large tsunamis occur every 2-3 years, with very large, damaging events happening every 15-20 years. The most devastating tsunami in terms of loss of life resulted from a magnitude 9 earthquake in Indonesia in 2004 (Figure 3.4.2), which created waves up to 33 m tall and left about 230,000 people dead in Indonesia, Thailand, and Sri Lanka. In 2011 a 9.0 magnitude earthquake in Japan triggered a tsunami up to 40.5 m high, which resulted in over 18,000 deaths. This earthquake also caused the Fukishima nuclear accident, and moved Japan about 8 inches closer to the U.S.

By Paul Webb, used under a CC-BY 4.0 international license. Download this book for free at https://rwu.pressbooks.pub/webboceanography/front-matter/preface/
Ions are not the only materials that are dissolved in seawater. The oceans also contain dissolved gases that are very important to living organisms, particularly oxygen (O2), carbon dioxide (CO2), and nitrogen (N2). Oxygen is required for respiration in marine plants, algae, and phytoplankton (the primary producers) and animals. Carbon dioxide is utilized by the primary producers to power photosynthesis, a byproduct of which is oxygen. Nitrogen gas dissolved in the ocean is fixed by bacteria and converted into the forms required for primary production, such as nitrate and nitrite.
All of these gases are found in the atmosphere, and can enter the ocean by dissolving into the water at the ocean’s surface. But the amount of each gas in air is very different from the amount found in the ocean (Table 5.4.1).
Table 5.4.1 Percentage of total gas in each compartment
| Air | Total Ocean | Surface Ocean | |
|---|---|---|---|
| N2 | 78% | 11% | 48% |
| O2 | 21% | 6% | 36% |
| CO2 | 0.04% | 83% | 15% |
The amount of each gas that can dissolve in the ocean depends on the solubility and saturation of the gas in water. Solubility refers to the amount of a dissolved gas that the water can hold under a particular set of conditions, which are usually defined as 0o C and 1 atmosphere of pressure. The solubility of a gas increases with increasing pressure, decreased temperature, and decreased salinity. Saturation refers to the amount of gas currently dissolved in the water, relative to the maximum possible content. If the water is undersaturated, more gas can dissolve. If the water is saturated or supersaturated, gas may be released. Most atmospheric gases are saturated in the ocean, but O2 and CO2 are not saturated because they are rapidly used by living organisms.
Oxygen
Typical oceanic dissolved oxygen profiles are shown in Figure 5.4.1. The shape of the profile is determined by the various processes that add or remove oxygen from the water at different depths.
Oxygen content is highest at the surface for two main reasons; this is where oxygen dissolves into the ocean from the atmosphere, and the surface water is where oxygen is produced by phytoplankton through photosynthesis. Respiration is also occurring in the surface waters, but the rate of photosynthetic oxygen production is greater than the rate of removal through respiration. It should be noted that even though dissolved oxygen is highest at the surface, there is still far less oxygen in the water than is found in the air. Well-oxygenated surface water may only contain around 8 mg O2/l, while the air contains 210 mg O2/l.
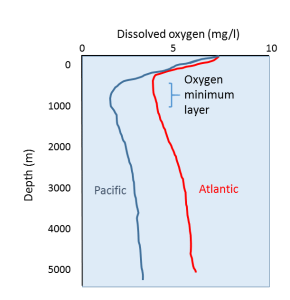
As depth increases, dissolved oxygen declines, reaching a minimum between a few hundred meters and 1000 m deep, the aptly-named oxygen minimum layer. At these depths and below, the water is too far removed from the surface for any atmospheric exchange, and there is not enough light to support photosynthesis, so there is little if any oxygen added to the water. At the same time, oxygen is removed from the water through the respiration of deep water organisms, and the decomposition of organic material by bacteria as it sinks to depth.
Below the oxygen minimum layer there is often an increase in dissolved oxygen at the greatest depths (Figures 5.4.1, 5.4.2). This bottom water is usually colder than the surface water and is under enormous pressure; as stated above, lower temperatures and higher pressure increase the solubility of dissolved gases. But there is another reason that bottom water contains more oxygen than mid-water depths that has to do with the way water circulates throughout the deep ocean (see section 7.8). In polar regions, the cold surface water absorbs lots of oxygen. This cold, oxygen-rich water sinks to the bottom due to its high density, taking the oxygen with it. The oxygen-rich bottom water will then spend the next thousand years or so moving over the seafloor throughout the major ocean basins. This deep water circulation is the source of oxygen for bottom-dwelling (benthic) organisms. The oxygen-rich bottom water forms in the polar regions of the Atlantic, and slowly makes its way to the Pacific, with oxygen being removed for respiration along the way. This is why dissolved oxygen levels in Pacific deep water are generally lower than in the Atlantic (Figure 5.4.1).
Areas where dissolved oxygen levels are too low to support most life are referred to as hypoxic zones (they are experiencing hypoxia, or low oxygen). Hypoxia is usually defined as oxygen levels below 2 mg/L. Anoxic zones (anoxia = without oxygen) show more severe forms of hypoxia, with oxygen below 0.5 mg/L. Some parts of the oceans may experience seasonal or temporary periods of hypoxia, while in other areas these conditions may last much longer. These hypoxic conditions often lead to mass die-offs of marine organisms who struggle to survive without sufficient oxygen.
Additional links for more information:
- NOAA site on oceanic hypoxic zones: http://oceanservice.noaa.gov/hazards/hypoxia/

