39 5.8 Sound
Sound is a form of energy transmitted through pressure waves; longitudinal or compressional waves similar to the seismic P-waves. With ocean sounds, the energy is transmitted via water molecules vibrating back and forth parallel to the direction of the sound wave, and passing on the energy to adjacent molecules. Therefore, sound travels faster and more efficiently when the molecules are closer together and are better able to transfer their energy to neighboring particles. In other words, sound travels faster through denser materials. Since water is much denser than air, the speed of sound in water (about 1500 m/s) is approximately five times faster than the speed in air (around 330 m/s). This helps explain why we sometimes have difficulty localizing the source of a sound that we hear underwater. We localize sound sources when our brains detect the tiny differences in the time of arrival of sounds reaching our ears. A sound coming from our left will reach our left ear a fraction of a second before reaching our right ear. Our brains can process that small difference in time of arrival to recognize the direction from which the sound came. In water, the sound is so much faster that the difference in arrival time between our ears becomes too small for us to interpret, and we lose the ability to localize the source.
However, as with sound in air, the speed of sound in the ocean is not constant; it is influenced by a number of variables including temperature, salinity, and pressure, and an increase in any of these factors will lead to an increase in the speed of sound. We have seen that these variables change with depth and location; so to will the speed of sound differ in different regions of the ocean.
To examine the way the speed of sound changes as a function of depth, we need to consider the vertical profiles for temperature and pressure. At the surface, the pressure is low, but the temperature is at its highest point in the water column. The temperature effects dominate at the surface, so the speed of sound is fast in surface waters. As depth increases, the temperature and the speed of sound decline. Near the bottom, the extreme pressure dominates, and even though temperatures are low, the speed of sound increases with depth. At moderate depths (between a few hundred and one thousand meters) there is a zone where both temperature and pressure are relatively low, so the speed of sound is at a minimum. This zone of minimum speed is called the SOFAR channel (Sound Fixing And Ranging) or the Deep Sound Channel (Figure 5.8.1).
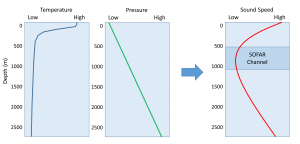
The SOFAR channel is important because sounds produced in that region can be propagated over very long distances with little attenuation (loss of energy). Sound waves produced in the channel radiate out in all directions. Waves that travel into shallower or deeper water outside of the sound channel are entering a region of faster sound transmission. As we saw with seismic waves, when these sound waves encounter a region of differing transmission speed, the waves tend to be refracted or bent back towards the region of lower speed. As a result, sound waves moving from the SOFAR channel into shallower water will be refracted back towards the channel. As the sound waves go deeper below the channel, they will be refracted upwards, back into the channel and the region of slower speed. In this way, much of the sound does not dissipate out into the water in all directions, but instead is trapped within the channel, and can travel very long distances with little loss of energy (Figure 5.8.2).
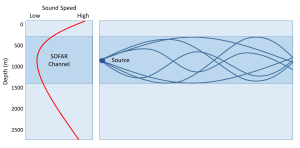
There are several practical applications of the SOFAR channel. Baleen whales are thought to use the SOFAR channel to communicate with each other over long distances of hundreds to thousands of kilometers. Their vocalizations are very loud and are low frequency calls, which travel farther than high frequency sounds in the oceans. The military has been able to track submarines using the SOFAR channel, and during World War II it was used to locate downed pilots or missing ships and planes. A stranded pilot could drop a small device into the water, and once it sank into the SOFAR channel it would explode, creating a sound that could be heard at multiple listening stations. Using the time of arrival of the sound at the various receivers, the location of the source could be determined through triangulation. In the 1990s it was suggested that the SOFAR channel could be used to monitor global ocean temperatures. A project known as ATOC (Acoustic Thermometry of Ocean Climate) was proposed where loud, low frequency sounds produced near Hawaii and California would travel through the SOFAR channel to receiving stations around the Pacific. By monitoring the time it took for the sounds to reach the receivers, scientists could monitor changes in ocean temperatures on a global scale, as sounds would move faster through a warming ocean.
Since sound travels better through water than air, the energy required to transmit a given sound wave is higher in air than in water. The energy, or intensity (loudness) of a sound is measured on the decibel (dB) scale. It turns out that it takes about 61 times more energy to transmit a sound through air than through water. Because of this energy difference, there is a 61 dB difference between sounds transmitted through air and water, such that a sound intensity of 120 dB in water would be equivalent to an intensity of about 60 dB in air. This should be kept in mind when trying to compare sounds in the ocean with sounds in the air. A sound of 130 dB in air is about equivalent to standing 100 m from a jet engine at takeoff. A sound of 130 dB in water is equivalent to about 70 dB in air, which is the intensity of the sound of a vacuum cleaner. It should also be pointed out that on the dB scale, an increase of 10 dB means the sound is 10 times louder. In other words, 20 dB is 10 x louder than 10 dB, while 30 dB is 100 x louder than 10 dB.
- Discovery of Sound in the Sea website: http://www.dosits.org/
All of the salts and ions that dissolve in seawater contribute to its overall salinity. Salinity of seawater is usually expressed as the grams of salt per kilogram (1000 g) of seawater. On average, about 35 g of salt is present in each 1 kg of seawater, so we say that the average salinity of the ocean salinity is 35 parts per thousand (ppt). Note that 35 ppt is equivalent to 3.5% (parts per hundred). Some sources now use practical salinity units (PSU) to express salinity values, where 1 PSU = 1 ppt. The units are not included, so we can refer simply to a salinity of 35.
Many different substances are dissolved in the ocean, but six ions comprise about 99.4% of all the dissolved ions in seawater. These six major ions are (Table 5.3.1):
Table 5.3.1 The six major ions in seawater
| g/kg in seawater | % of ions by weight | |
|---|---|---|
| Chloride Cl- | 19.35 | 55.07% |
| Sodium Na+ | 10.76 | 30.6% |
| Sulfate SO42- | 2.71 | 7.72% |
| Magnesium Mg2+ | 1.29 | 3.68% |
| Calcium Ca2+ | 0.41 | 1.17% |
| Potassium K+ | 0.39 | 1.1% |
| 99.36% |
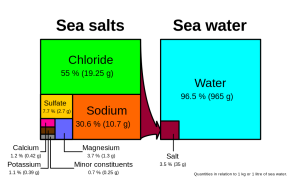
Chloride and sodium, the components of table salt (sodium chloride NaCl), make up over 85% of the ions in the ocean, which is why seawater tastes salty (Figure 5.3.1). In addition to the major constituents, there are numerous minor constituents; radionucleotides, organic compounds, metals etc. These minor constituents are found in concentrations of ppm (parts per million) or ppb (parts per billion), unlike the major ions that are far more abundant (ppt) (Table 5.3.2). To put this into perspective, 1 ppm = 1 mg/kg, or the equivalent of 1 teaspoon of sugar dissolved in 14,000 cans of soda. 1 ppb = 1 μg/kg, or the equivalent of 1 teaspoon of a substance dissolved in five Olympic-sized swimming pools! These minor constituents represent numerous substances, but together they make up less than 1% of the ions in the seawater. Some of these may be important as minerals and trace elements vital to living organisms, but they don’t have much impact on the overall salinity. But given the vast size of the oceans, even materials found in trace abundance can represent fairly large reservoirs. For example gold is a trace element in seawater, found in concentrations of parts per trillion, yet if you could extract all of the gold in just one km3 of seawater, it would be worth about $20 million!
Table 5.3.2 Concentrations of some minor elements in seawater
| g/kg in seawater | g/kg in seawater | ||
|---|---|---|---|
| Carbon | 0.028 | Iron | 2 x 10-6 |
| Nitrogen | 0.0115 | Manganese | 2 x 10-7 |
| Oxygen | 0.006 | Copper | 1 x 10-7 |
| Silicon | 0.002 | Mercury | 3 x 10-8 |
| Phosphorous | 6 x 10-5 | Gold | 4 x 10-9 |
| Uranium | 3.2 x 10-6 | Lead | 5 x 10-10 |
| Aluminum | 2 x 10-6 | Radon | 6 x 10-19 |
Because the six major ions in seawater comprise over 99% of the total salinity, changes in abundance of the minor constituents have little effect on overall salinity. Furthermore, the rule of constant proportions states that even though the absolute salinity of ocean water might differ in different places, the relative proportions of the six major ions within that water are always constant. For example, no matter the total salinity of a seawater sample, 55% of the total salinity will be due to chloride, 30% due to sodium, and so on. Since the proportion of these major ions does not change, we call these conservative ions.
Given these constant proportions, in order to calculate total salinity you can simply measure the concentration of just one of the major ions and use that value to calculate the rest. Traditionally chloride has been the ion measured because it is the most abundant, and thus the simplest to measure accurately. Multiplying the concentration of chloride by 1.8 gives the total salinity. For example, looking at Figure 5.3.1, 19.25 g/kg (ppt) chloride x 1.8 = 35 ppt. Today, for rapid measurements of salinity, electrical conductivity is often used rather than determining chloride concentrations (see box below).
Measuring salinity
There are a number of methods available for measuring the salinity of water. The most precise measurements utilize direct chemical analysis of the seawater in a lab setting, but there are a number of ways to get immediate salinity measurements in the field. For a quick estimate of salinity, a hand-held refractometer can be used (right).

This instrument measures the degree of bending, or refraction, of light rays as they pass through a fluid. The greater the amount of dissolved salts in the sample, the greater the degree of light refraction. The observer traps a drop of water on the blue screen, and looks through the eyepiece. The dividing line between the blue and white sections of the scale (inset) can be used to read the salinity.
For more accurate measurements, most oceanographers use an instrument that measures electrical conductivity. An electrical current is passed between two electrodes immersed in water, and the higher the salinity, the more readily the current will be conducted (the ions in seawater conduct electrical currents). Conductivity probes are often bundled into an instrument called a CTD, which stands for Conductivity, Temperature, and Depth, which are the most commonly-measured parameters. Modern CTDs can be outfitted with an array of probes measuring parameters like light, turbidity (water clarity), dissolved gases etc. CTDs can be large instruments (below), but small hand-held salinity probes are also widely available.

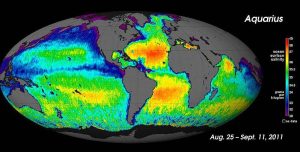
For large-scale salinity measurements, oceanographers can use satellites, such as the Aquarius satellite, which was able to measure surface salinity differences as small as 0.2 PSU as it mapped the ocean surface every seven days (below).
It is important to be aware that while the rule of constant proportions applies to most of the ocean, there may be certain coastal areas where lots of river discharge may alter these proportions slightly. Furthermore, it is important to remember that the rule of constant proportions only applies to the major ions. The proportions of the minor ions may fluctuate, but remember that they make a very minor contribution to overall salinity. Because the concentrations of the minor ions are not constant, these are referred to as non-conservative ions.
Why are the major ions found in constant proportions? There is constant input of ions from river runoff and other processes, usually in very different proportions from what is found in the ocean. So why don’t the proportions in the oceans change? Most of the ions discharged by rivers have fairly low residence times (see section 5.2) compared to ions in seawater, usually because they are used in biological processes. These low residence times do not allow the ions to accumulate and alter salinity. Also, the mixing time of the world ocean is around 1000 years, which is very short compared to the residence times of the major ions, which may be tens of millions of years long. So during the residence time of a single ion the ocean has mixed numerous times, and the major ions have become evenly distributed throughout the ocean.
Variations in Salinity
Total salinity in the open ocean averages 33-37 ppt, but it can vary significantly in different locations. But since the major ion proportions are constant, the regional salinity differences must be due more to water input and removal rather than the addition or removal of ions. Fresh water input comes through processes like precipitation, runoff from land, and melting ice. Fresh water removal primarily comes from evaporation and freezing (when seawater freezes, the resulting ice is mostly fresh water and the salts are excluded, making the remaining water even saltier). So differences in rates of precipitation, evaporation, river discharge, and ice formation play a significant role in regional salinity variations. For example, the Baltic Sea has a very low surface salinity of around 10 ppt, because it is a mostly enclosed body of water with lots of river input. Conversely, the Red Sea is very salty (around 40 ppt), due to the lack of precipitation and the hot environment which leads to high levels of evaporation.
One of the saltiest large bodies of water on Earth is the Dead Sea, between Israel and Jordan. Salinity in the Dead Sea is around 330 ppt, which is almost ten times saltier than the ocean. This extremely high salinity is a result of the hot, arid conditions in the Middle East that lead to high rates of evaporation. In addition, in the 1950s the flow from the Jordan River was diverted away from the Dead Sea, so there is no longer significant fresh water input. With no input and high evaporation, the water level in the Dead Sea is receding at a rate of about 1 m per year. The high salinity makes the water very dense, which creates buoyant forces that allow people to easily float at the surface. But the high salinity also means that the water is too salty for most living organisms, so only microbes are able to call it home; hence the name the Dead Sea. But as salty as the Dead Sea may be, it is not the saltiest body of water on Earth. That distinction currently belongs to Gaet’ale Pond in Ethiopia, with a salinity of 433 ppt!
Latitudinal Variations
While local conditions are important for determining salinity patterns in any single location, there are some global patterns that bear further investigation. Temperature is highest at the equator, and lowest near the poles, so we would expect higher rates of evaporation, and therefore higher salinity, in equatorial regions (Figure 5.3.2). This is generally the case, but in the figure below salinity right along the equator seems to be a little lower than at slightly higher latitudes. This is because equatorial regions also get a high volume of rain on a regular basis, which dilutes the surface water along the equator. So the higher salinities are found at subtropical, warm latitudes with high evaporation and less precipitation. At the poles there is little evaporation, which, coupled with ice and snow melting, produces a relatively low surface salinity. The image below shows high salinity in the Mediterranean Sea; this is located in a warm region with high evaporation, and the sea is largely isolated from mixing with the rest of the North Atlantic water, leading to high salinity. Lower salinities, such as those around southeast Asia, are the result of precipitation and high volumes of river input.
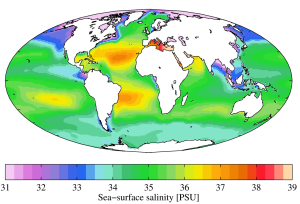
Figure 5.3.3 shows the mean global differences between evaporation and precipitation (evaporation - precipitation). Green colors represent areas where precipitation exceeds evaporation, while brown regions are where evaporation is greater than precipitations. Note the correlation between precipitation, evaporation, and surface salinity as seen in Figure 5.3.2.

Vertical Variation
In addition to geographical variation in salinity, there are also changes in salinity with depth. As we have seen, most differences in salinity are due to variations in evaporation, precipitation, runoff, and ice cover. All of these process occur at the ocean surface, not at depth, so the most pronounced differences in salinity should be found in surface waters. Salinity in deeper water remains relatively uniform, as it is unaffected by these surface processes. Some representative salinity profiles are shown in Figure 5.3.4. At the surface, the top 200 m or so show relatively uniform salinity in what is called the mixed layer. Winds, waves, and surface currents stir up the surface water, causing a great deal of mixing in this layer and fairly uniform salinity conditions. Below the mixed layer is an area of rapid salinity change over a small change in depth. This zone of rapid change is called the halocline, and it represents a transition between the mixed layer and the deep ocean. Below the halocline, salinity may show little variation down to the seafloor, as this region is far removed from the surface processes that impact salinity. In the figure below, note the low surface salinity at high latitudes, and higher surface salinity at low latitudes as discussed above. Yet despite the surface differences, salinity at depth in both locations may be very similar.


