9.6 Parkinson’s Disease
Open Resources for Nursing (Open RN)
Parkinson’s disease (PD) is a common, progressive neurological movement disorder of older adults that eventually leads to disability. Parkinson’s disease can be divided into five stages:
- Stage 1 (Initial): Unilateral limb involvement, hand and arm trembling, and mild weakness
- Stage 2 (Mild Stage): Bilateral limb involvement, resting tremor, bradykinesia (slow, shuffling gait), and mask-like facial expression
- Stage 3 (Moderate): Postural instability, increased gait irregularities, and pill-rolling tremor
- Stage 4 (Severe): Akinesia (the inability to voluntarily move muscles) and rigidity
- Stage 5 (Total Dependence): Total reliance on caregivers for ADLs
Pathophysiology
Parkinson’s disease is associated with decreased levels of dopamine. In normal brain activity, the excitatory neurotransmitter acetylcholine and the inhibitory neurotransmitter dopamine relay messages to the motor centers in the brain where control of motor movement occurs. When these neurotransmitters are balanced, there is refined and coordinated movement, such as picking up a pencil and writing. However, decreased dopamine levels cause an imbalance with the excitatory neurotransmitter acetylcholine, affecting voluntary movement. The clinical symptoms of PD typically do not appear until dopamine levels decrease by 80%. The loss of dopamine also reduces the sympathetic nervous system stimulation of the heart and blood vessels, which can result in orthostatic hypotension, drooling, nocturia, and other autonomic symptoms.
Risk Factors
The cause of Parkinson’s disease is not known, but risk factors include genetic predisposition, viral infections, atherosclerosis, head trauma, long-term use of antipsychotic medications, and environmental exposures. PD is typically diagnosed in clients in their sixties or seventies, but in some cases it can occur as early as 30 years of age. PD tends to affect more men than women, and there is increased risk with family history in first-degree relatives.
Health Disparities Related to Parkinson Disease
A recent study identified crucial gaps in care for people diagnosed with Parkinson’s disease in the United States. The greatest disparities in care occur in women, people of color (those who identify as Asian, Black, Hispanic and Native American), and residents of rural areas. These disparities include lack of access to movement disorder specialists (expertly trained neurologists who can tailor treatments to each client), treatment of associated depression from mental health professionals, and access to supplemental therapies such as physical and occupational therapy, speech-language therapy, and mental health services.[1]
Assessment
PD typically has a gradual onset with progression of symptoms. Four primary symptoms of PD include the following[2]:
- Resting tremor
- Muscle rigidity
- Bradykinesia or akinesia
- Postural instability
Although PD is not a form of dementia, cognitive changes can occur later in the disease process, such as dementia or psychosis.
The most common reason people initially seek medical care is due to a resting tremor. Resting tremors disappear with purposeful, voluntary movement, but become apparent when the person’s extremities are motionless. Eventually, the tremor becomes a repetitive, constant, slow-turning motion between the forearm, hand, and thumb, commonly known as pill-rolling, as if the person is rolling a pill between the fingers. The pill-rolling tremor is present at rest and often increases as the client walks, concentrates, or experiences anxiety.[3]
View a supplementary YouTube video[4] on pill rolling: Pill Rolling Resting Tremor | Parkinson Disease.
Muscle rigidity refers to resistance to passive limb movement. Cogwheel rigidity is seen frequently with PD, characterized by a jerky rhythmic tone on passive muscle stretching. The muscles remain constantly tense and contracted so that the person may feel achy and stiff. The rigidity becomes evident when another person tries to move the individual’s arm, which will move only in short, jerky movements. Muscle rigidity is present early in the disease and progresses over time, causing the client to require assistance with ADLs and ambulation. The facial muscles may also experience muscle rigidity, causing a mask-like appearance with wide-open, fixed, staring eyes. In later stages of PD, muscle rigidity can lead to difficulty with chewing and swallowing as the pharyngeal muscles become involved, placing the client at risk for aspiration and malnutrition. Uncontrolled changes in speech patterns are also common symptoms. Clients may speak very softly, slur or repeat words, hesitate before speaking, project a monotone voice, or exhibit a rapid speech pattern.[5]
Bradykinesia refers to slowness of initiation of movement, and akinesia refers to a lack of voluntary movement. For example, clients may have difficulty initiating movements, such as rising from a chair to stand.[6]
Postural disturbances become apparent as loss of reflexes occur. A client with PD typically stands with their head bent forward and walks with a propulsive gait. This characteristic posture is caused by the forward flexion of the neck, hips, knees, and elbows. They tend to compensate for this postural disturbance by moving their feet forward under the body’s center of gravity, referred to as shuffling gait. Clients with PD are at high risk for falls due to this postural disturbance and lack of balance.[7] See Figure 9.17[8] for illustrations of postural disturbances of clients with various stages of PD.
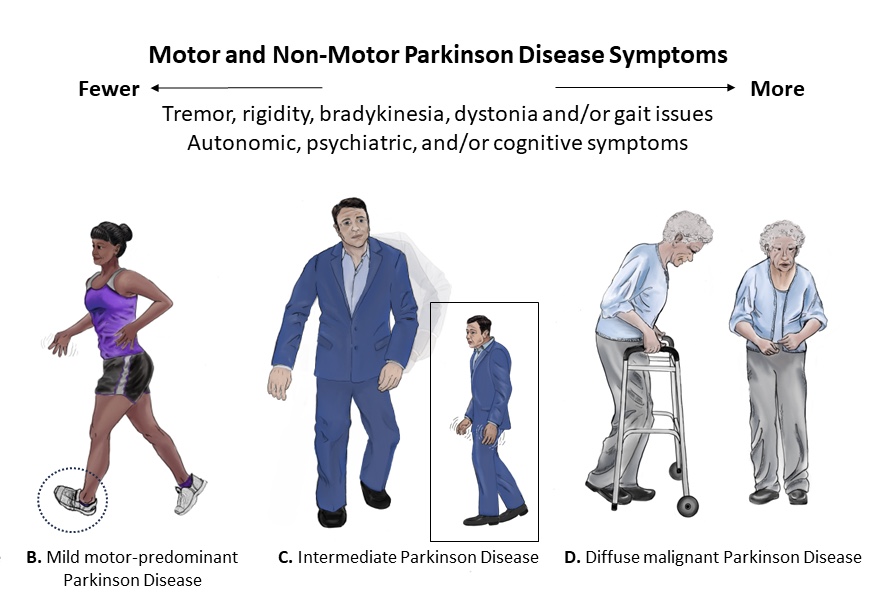
View an animation[9] of the characteristic gait of a person with Parkinson’s disease: Abnormal Gait Exam: Parkinsonian Gait Demonstration.
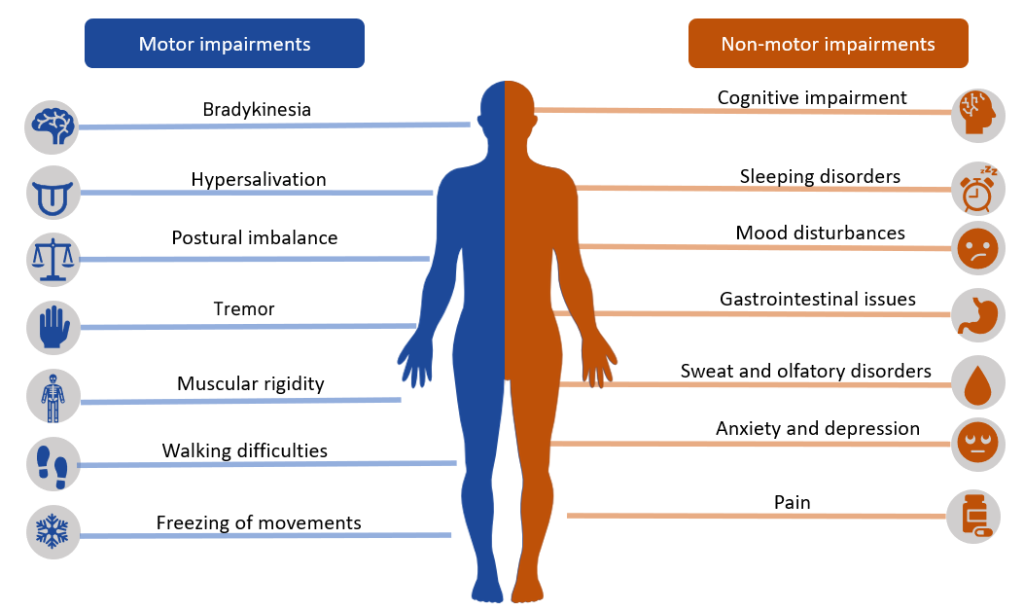
In addition to the four classic symptoms of PD, autonomic symptoms such as excessive sweating, orthostatic hypotension, urinary retention, constipation, and sexual dysfunction may also occur. Mood and personality changes may occur, such as depression, psychosis, dementia, sleep disturbances, and auditory or visual hallucinations. Cognitive changes can also affect the judgment and decision-making of a person with PD. See Figure 9.18[10] for an illustration of motor and nonmotor impairments that may occur in clients with Parkinson’s disease.
Common Labs and Diagnostic Tests
There are no diagnostic tests that specifically diagnose Parkinson disease. Providers diagnose PD if the client has at least two of the four cardinal symptoms previously discussed. Other potential neurologic diseases are eliminated with the following tests:
- Blood tests may be ordered to rule out other disorders that may be causing the symptoms.
- Single photon emission computed tomography (SPECT) may be ordered to rule out other nervous system disorders. It can also detect a loss of dopamine-producing neurons.
- Analysis of cerebrospinal fluid may show a decrease in dopamine levels.
- Brain scans such as CTs and MRIs are performed to rule out other nervous system disorders.
Nursing Diagnoses
Priority nursing diagnoses for clients with PD focus on impaired mobility, cognitive function, communication, and gastrointestinal function. Common nursing diagnoses include the following[11]:
- Impaired Physical Mobility
- Impaired Swallowing
- Chronic Confusion
- Impaired Memory
- Activity Intolerance
- Impaired Verbal Communication
- Risk for Falls
- Constipation
Outcome Identification
Overall goals for clients with Parkinson’s disease include improving mobility, functioning, and independence in performing ADLs; preventing falls and injury; promoting optimal bowel elimination; maintaining acceptable nutritional status; achieving effective communication and positive coping mechanisms; and teaching the client and their family on the disease process and self-care strategies to manage symptoms and improve the overall quality of life. Sample outcome criteria include the following:
- The client will remain free of complications related to immobility.
- The client will maintain adequate nutritional intake with a BMI in the normal range.
- The client will not experience aspiration.
- The client will remain free of injuries.
- The client will use methods of communication to make needs known to caregivers after the teaching session.
- The client will verbalize three strategies to effectively cope with chronic disease after the teaching session.
Interventions
Medical Interventions
Because there is no cure for PD, medical management focuses on alleviating symptoms and improving the client’s quality of life. Medical interventions are tailored to an individual’s symptoms, disease severity, age, and lifestyle.
Medication Therapy
Medications are the most common medical intervention, with the goal to increase dopamine levels or mimic dopamine effects in the brain. Medications must be closely monitored to prevent long-term side effects and complications.
Levodopa is the most effective medication used to treat PD. Levodopa is converted to dopamine in the brain and provides some symptom relief. Levodopa is commonly prescribed in combination with carbidopa to maximize its benefits. These medications are most beneficial in the first few years of treatment. However, over time, their benefits begin to lessen, and side effects such as confusion, hallucinations, depression, and sleep disturbances can develop.
After approximately 5 to 10 years of chronic use, the client may develop undesirable side effects from these medications referred to as dyskinesia. Dyskinesia refers to abnormal involuntary movements such as facial grimacing, rhythmic jerking movements of hands, lip-smacking, and head bobbing. Clients on chronic medications are also at risk for drug tolerance, where therapeutic effects do not last as long as they did previously. Treatment of dyskinesia involves reducing the dose, changing the drug or frequency of administration, or requesting a drug holiday. During a drug holiday, the client does not receive any medication for PD for up to ten days. Additional medications such as baclofen for muscle spasms or sublingual atropine for excessive drooling may be administered. During the drug holiday, the client is closely monitored for dyskinesia and PD symptoms, and the symptoms are documented.
Other medications prescribed for symptom relief include MAO-B inhibitors, anticholinergics, antivirals, dopamine agonists, COMT inhibitors, and antidepressants. See Table 9.6 for an overview of medications used to treat PD.
Table 9.6. Antiparkinson Medications[12]
| Medication | Nursing Considerations and Side Effects |
|---|---|
| Antiparkinson’s Agent
Carbidopa/Levodopa |
Initial side effects of levodopa-carbidopa may include nausea, low blood pressure, restlessness, and drowsiness.
Side effects of long-term or extended use of levodopa may include hallucinations, psychosis, and dyskinesia. |
| Anticholinergic Therapy
Trihexyphenidyl Hydrochloride Benztropine Mesylate |
Controls tremor and assists with drooling by counteracting the neurotransmitter acetylcholine.
Typically, poorly tolerated in older adults. May increase intraocular pressure. Male clients with BPH are monitored for urinary retention. |
| Antiviral Therapy
Amantadine Hydrochloride |
Improves symptoms related to akinesia, and tremor.
Usually well-tolerated, but side effects may include mood changes, confusion, depression, hallucinations, orthostatic hypotension, and headache. |
| Dopamine Agonists
Bromocriptine Mesylate Pergolide Ropinirole Hydrochloride Pramipexole |
Useful before initiating carbidopa or levodopa therapy or when added after carbidopa or levodopa lose their effectiveness.
Adverse effects include lightheadedness, hypotension, diarrhea, and psychiatric effects. |
| Catechol-O-Methyltransferase (COMT) Inhibitors
Entacapone Tolcapone |
May increase duration of action when given in combination with carbidopa or levodopa.
Block an enzyme that breaks down levodopa, making it more available to convert to dopamine in the brain. |
| Tricyclic Antidepressants
Amitriptyline Hydrochloride |
Used for the anticholinergic and antidepressant effects.
Dosing is usually ⅓ to ½ the typical dose in clients with PD. |
| Selective Serotonin Reuptake Inhibitors (SSRIs)
Fluoxetine Hydrochloride |
Effective for treating depression but may aggravate symptoms of Parkinson’s disease. |
| Atypical Antidepressants
Bupropion Hydrochloride |
Effective for treating depression but may aggravate symptoms of Parkinson’s disease. |
| Monoamine Oxidase Inhibitors (MAOIs)
Selegiline Rasagiline Zydis Selegiline HCL |
Used in combination with a dopamine agonist to postpone the initiation of carbidopa or levodopa therapy. Adverse effects are similar to that of levodopa.
Teach clients taking MAOIs to avoid foods, beverages, and drugs that contain tyramine, such as cheese; aged, smoked, or cured foods; and not to drink beer or red wine to prevent headache and life-threatening hypertension. If the drug is discontinued, follow restrictions for 14 days after the last dose. |
Physical, Occupational, and Speech Therapy
Because shuffling gait, postural instability, and other muscle problems are major problems for clients with PD, collaboration with physical and occupational therapists is important in early stages of the disease. Physical therapists plan and implement programs to prevent falling and maintain flexibility and mobility through range-of-motion exercises, muscle stretching, and physical activity. Teaching clients how to safely use ambulatory assistive devices is also provided. Occupational therapists teach clients how to use adaptive devices, such as specialized eating utensils. These rehabilitation services encourage clients to participate as much as possible in their ADLs while ensuring the environment is safe.
Speech and language pathologists (SLP) help treat clients with speech and swallowing difficulties. SLPs teach clients how to perform exercises to strengthen the muscles used for swallowing, speaking, and breathing. Depending on the client’s level of functioning, the SLP develops a communication plan and assists with alternative methods of communication such as a communication board, mechanical voice synthesizer, computer, or handheld mobile device.
Surgical Management
Surgery may be performed when medications fail to provide symptom management. Common surgeries are stereotactic pallidotomy and deep brain stimulation. During stereotactic pallidotomy, a probe is placed in the globus pallidus portion of the brain, destroying specific tissue and creating scar tissue. The scar tissue interrupts the overactive nerve signal transmission, reducing the brain activity in that area and helping to treat rigidity and reducing tremor.[13]
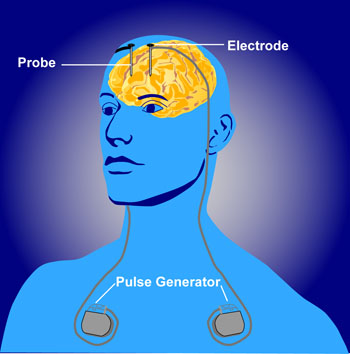
Deep brain stimulation involves implanting electrodes in the brain that are attached to a pulse generator. The generator device is placed under the skin and delivers an electric current to decrease dyskinesias and reduce the need for dopamine precursor medications. See Figure 9.19[14] for an illustration of deep brain stimulation.
Nutritional Management
Consultation with a registered dietician or nutritionist is helpful to evaluate the client’s food intake and their ability to swallow. If dysphagia is present, the SLP and dietician collaborate on the most appropriate diet for the client, such as a soft diet with thickened liquids or incorporation of supplement shakes. If the client is experiencing constipation, the dietician can also help adjust the diet.
Nursing Interventions
Nursing interventions follow a holistic approach when caring for clients with PD and play a crucial role in enhancing their quality of life. Interventions focus on enhancing mobility and self-care activities, improving communication, assisting with optimal nutrition intake, facilitating swallowing, and supporting effective coping strategies. Specific nursing interventions are summarized in the following box.
Nursing Interventions for Clients with Parkinson’s Disease[15]
- Administer prescribed medications on schedule to maintain consistent therapeutic levels. Monitor for side effects, especially orthostatic hypotension, acute confusion, and hallucinations.
- Initiate fall precautions according to agency policy.
- Allow the client time to perform ADLs and provide assistance only as needed to promote independence.
- Implement interventions that prevent complications related to immobility such as pressure injuries, constipation, and contractures.
- Monitor the client’s ability to chew and swallow, as well their food and fluid intake.
- Schedule appointments and activities late in morning or when clients are at their optimal levels of functioning to avoid rushing or fatigue.
- Collaborate with a dietician to provide nutritional, high-calorie foods to maintain strength and well-being.
- Allow the client extra time to respond to questions to promote effective communication. Encourage clients to speak slowly and clearly. Use alternative forms of communication, such as communication boards, as indicated by SLP.
- Collaborate with physical and occupational therapy to promote optimal mobility, physical activity, and independence in completing their ADLs.
- Focus on the client’s strengths, recognizing that PD can lower one’s self-esteem.
- Monitor for new signs of depression, anxiety, impaired cognition, insomnia, and sleep disturbances.
Health Teaching
Nurses provide health teaching to the client and their family members on the following topics:
- Instruct on safe medication administration and promptly report any adverse effects of medication, such as acute confusion and hallucinations.
- Teach how to prevent falls (remove rugs and clutter, use assistive devices).
- Teach the importance of focusing on the client’s abilities and strengths and offer positive reinforcement when goals are met.
- Encourage the family and client to work collaboratively with the rehabilitation team to maintain optimal level of functioning.
- Provide education regarding symptom management, for example, increasing fluids and fiber for issues with constipation.
- Instruct on sleep enhancement such as avoidance of alcohol and caffeine, darkening lighting, and bedtime routine.
- Teach family, along with the dietician, about healthy and appropriate food choices to promote optimal nutrition.
- Encourage family and caregivers that many times the client cannot control symptoms of anxiety or depression and to seek assistance from health care providers, case managers, and support groups.
- Refer clients and family to local and national Parkinson’s disease organizations to facilitate resources available for caregiver strain.
Caring for a client with PD can pose unique challenges for family members. It is helpful to establish a case manager to ensure the psychosocial and physical needs are addressed. A case manager or social worker can also assist the family with financial and health insurance issues, as well as prepare for respite or long-term care.
Evaluation
Evaluation of client outcomes refers to the process of determining whether or not client outcomes were met by the indicated time frame. This is done by reevaluating the client as a whole and determining if their outcomes have been met, partially met, or not met. If the client outcomes were not met in their entirety, the care plan should be revised and reimplemented. Evaluation of outcomes should occur each time the nurse assesses the client, examines new laboratory or diagnostic data, or interacts with a family member or other member of the client’s interdisciplinary team.
![]() RN Recap: Parkinson’s Disease
RN Recap: Parkinson’s Disease
View a brief YouTube video overview of Parkinson’s disease[16]:
- Parkinson’s Foundation. (2023, July 10). New Medicare study finds critical gaps and disparities in access to Parkinson’s care. https://www.parkinson.org/blog/awareness/care-access-disparities ↵
- National Institute of Neurological Disorders and Stroke. (n.d.). Parkinson’s disease. https://www.ninds.nih.gov/health-information/disorders/parkinsons-disease ↵
- National Institute of Neurological Disorders and Stroke. (n.d.). Parkinson’s disease. https://www.ninds.nih.gov/health-information/disorders/parkinsons-disease ↵
- Extensive Medicine. (2023, December 19). Pill rolling resting tremor | Parkinson disease. [Video]. YouTube. All rights reserved. https://www.youtube.com/watch?v=7Q1G3llwAPs ↵
- National Institute of Neurological Disorders and Stroke. (n.d.). Parkinson’s disease. https://www.ninds.nih.gov/health-information/disorders/parkinsons-disease ↵
- National Institute of Neurological Disorders and Stroke. (n.d.). Parkinson’s disease. https://www.ninds.nih.gov/health-information/disorders/parkinsons-disease ↵
- National Institute of Neurological Disorders and Stroke. (n.d.). Parkinson’s disease. https://www.ninds.nih.gov/health-information/disorders/parkinsons-disease ↵
- “Modern_Parkinson%27s_Disease_Image_Cropped.jpg by Erica Rodriguez (artist), Michael S. Okun (author), cropped by User:Dustfreeworld is in the Public Domain. ↵
- “Abnormal_Gait_Exam_Parkinsonian_Gait_Demonstration.gif” by unknown author, via MakeAGIF is in the Public Domain ↵
- "Bradykinesia" by Meredith Pomietlo is a derivative of “Parkinson_disease_symtpoms.png” by CarrotsMitHummus licensed under CC BY-SA 4.0 ↵
- Herdman, T. H., Kamitsuru, S., & Lopes, C. T. (Eds.). (2020). Nursing diagnoses: Definitions and classification, 2021-2023 (12th ed.). Thieme. ↵
- National Institute of Neurological Disorders and Stroke. (n.d.). Parkinson’s disease. https://www.ninds.nih.gov/health-information/disorders/parkinsons-disease ↵
- University of Washington Medicine - Neurological Surgery. (n.d.). Stereostatic treatments for Parkinson’s disease. https://neurosurgery.uw.edu/patients-and-family/what-we-treat/treatments/stereotactic-treatments-parkinsons-disease ↵
- “Deep_brain_stimulation.jpg“ by http://www.nimh.nih.gov/health/topics/brain-stimulation-therapies/brain-stimulation-therapies.shtml is in the Public Domain ↵
- Nunes, S. F. L., Alvarez, A. M., & Valcarenghi, R. V. (2022). Parkinson’s disease in primary health care and nursing care: A scoping review. Revista da Escola de Enfermagem da USP. https://doi.org/10.1590/1980-220x-reeusp-2021-0367 ↵
- Open RN Project. (2024, June 23). Health Alterations - Chapter 9 - Parkinson's disease [Video]. You Tube. CC BY-NC 4.0 https://youtu.be/aeS6N_WwvEc?si=MLKhb_2M4Xl6Qpog ↵
Blood glucose monitoring is performed on patients with diabetes mellitus and other conditions that cause elevated blood sugar levels. Diabetes mellitus is a common medical condition that affects the body’s ability to produce insulin in the pancreas and use insulin at the cellular level. There are two types of diabetes mellitus, type 1 and type 2. Type 1 diabetes mellitus is an autoimmune disease that damages the beta cells of the pancreas so they do not produce insulin; thus, synthetic insulin must be administered by injection or infusion. It typically begins in childhood or adolescence. Type 2 diabetes mellitus accounts for approximately 95 percent of all cases and is highly correlated with obesity and inactivity. During type 2 diabetes, the cells of the body become resistant to the effects of insulin, and the pancreas increases its production of insulin. However, over time, the pancreas may no longer be able to produce insulin. In many cases, type 2 diabetes can be managed by moderate weight loss, regular physical activity, and a healthy diet. However, if blood glucose levels cannot be controlled with healthy lifestyle choices, oral diabetic medication is prescribed and eventually, the administration of insulin may be required.[1] Prediabetes is a medical condition where blood sugar levels are higher than normal, but not high enough yet to be diagnosed as type 2 diabetes. Approximately one in three American adults have prediabetes. Gestational diabetes is a type of diabetes that occurs during pregnancy in women who did not have diabetes before they were pregnant.
Diabetic patients require frequent blood glucose monitoring to administer customized medication therapy to prevent long-term complications from occurring. Hospitalized patients who do not have diabetes may also require frequent blood glucose monitoring due to elevations that can occur as a result of the stress of hospitalization, surgical procedures, and side effects of medications. Additionally, patients receiving enteral feedings typically have their blood glucose monitored every six hours. Health care providers prescribe the frequency of blood glucose monitoring; testing is typically performed before meals and at bedtime. For some patients, a standardized sliding-scale insulin protocol may be prescribed with instructions on the medication administration record (MAR) for administration of insulin based on their blood glucose results.[2],[3] See Table 19.2 for an example of a sliding-scale insulin protocol.
Table 19.2 Sample Sliding-Scale Insulin Protocol
Instructions: Check patient’s blood sugar before meals, at bedtime, and as needed for symptoms of hypoglycemia or hyperglycemia. Use the following table to administer insulin lispro PRN.
| Blood Sugar Range | Lispro Insulin Instructions |
|---|---|
| Less than 70 | Hold all insulin and initiate hypoglycemia protocol. |
| 70-150 | 0 units |
| 151-174 | 2 units |
| 175-199 | 4 units |
| 200-224 | 6 units |
| 225-249 | 8 units |
| 250-274 | 10 units |
| 275-299 | 12 units |
| Greater than 300 | Administer 14 units and call the provider. |
Hypoglycemia
When caring for patients with diabetes mellitus and monitoring their blood glucose readings, it is important to continually monitor for signs of hypoglycemia. Hypoglycemia is defined as blood sugar readings less than 70 and signs and symptoms such as the following:
- Shakiness
- Feeling nervous or anxious
- Sweating, chills, and clamminess
- Irritability or impatience
- Confusion
- Fast heartbeat
- Feeling light-headed or dizzy
- Hunger
- Nausea
- Color draining from the skin (pallor)
- Feeling sleepy
- Feeling weak or having no energy
- Blurred/impaired vision
- Tingling or numbness in the lips, tongue, or cheeks
- Headaches
- Coordination problems or clumsiness
- Nightmares or crying out during sleep
- Seizures[4]
A low blood sugar level triggers the release of epinephrine (adrenaline), the “fight-or-flight” hormone. Epinephrine causes the symptoms of hypoglycemia such as a rapid heartbeat, sweating, and anxiety. If a patient’s blood sugar level continues to drop, the brain has impaired functioning. This may lead to seizures and a coma.[5]
If a nurse suspects hypoglycemia is occurring, a blood sugar reading should be obtained, and appropriate actions taken. Most agencies have a hypoglycemia protocol based on the “15-15 Rule.” The 15-15 Rule is to provide 15 grams of carbohydrate and recheck the blood glucose after 15 minutes. If the reading is still below 70 mg/dL, another serving of 15 grams of carbohydrate should be provided and the process continued until the blood sugar is above 70 mg/dL. Fifteen grams of carbohydrate includes options like 4 ounces of juice or regular soda, hard candy, or glucose tablets. If a patient is experiencing severe hypoglycemia and cannot swallow, a glucagon injection or intravenous administration of dextrose may be required.[6]
Hyperglycemia
Hyperglycemia is defined as elevated blood glucose and often causes signs and symptoms such as frequent urination and increased thirst. Hyperglycemia occurs when the patient’s body does not produce enough insulin or cannot use the insulin properly at the cellular level. There are many potential causes of hyperglycemia, such as not receiving enough medication to effectively control blood glucose, eating more than planned, exercising less than planned, or increased stress from an illness, surgery, hospitalization, or other life events.
If a patient's blood glucose is greater than 240 mg/dL, their urine is typically checked for ketones. Ketones indicate a condition called ketoacidosis may be occurring. Ketoacidosis occurs in patients whose pancreas is no longer creating insulin, so fats are broken down for energy and waste products called ketones are produced. If the kidneys cannot effectively eliminate ketones in the urine, they build up in the blood and cause ketoacidosis. Ketoacidosis is a life-threatening condition that requires immediate notification of the provider for treatment. Symptoms of ketoacidosis include fruity-smelling breath, nausea, vomiting, very dry mouth, and shortness of breath. Treatment of ketoacidosis often requires the administration of intravenous insulin while the patient is closely monitored in a critical care inpatient unit.[7]
For more information about diabetes mellitus, measuring blood sugar levels, and diabetic medications, visit the "Endocrine" chapter in Open RN Nursing Pharmacology.
Glucometer Use
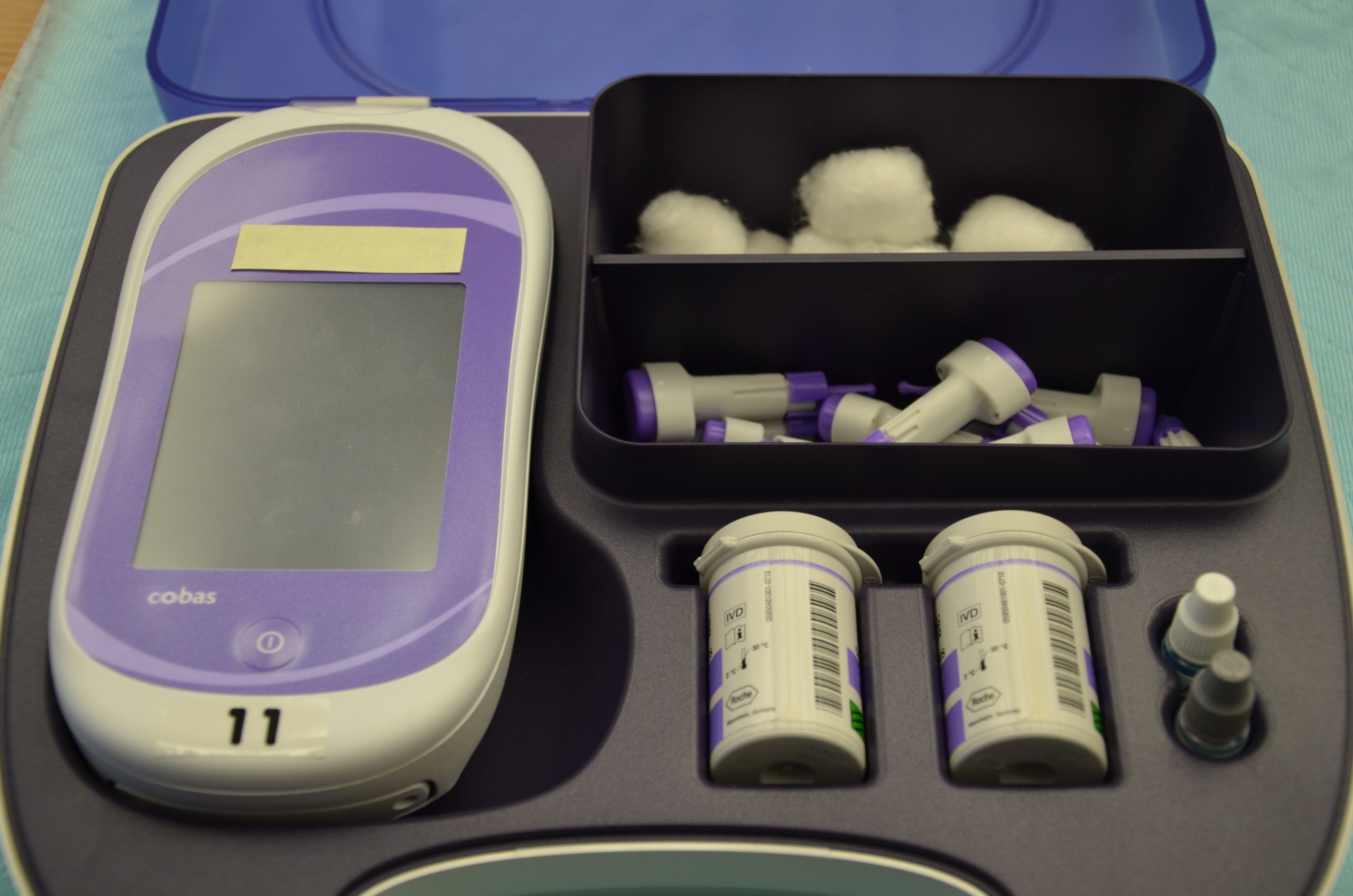
It is typically the responsibility of a nurse to perform bedside blood glucose readings, but in some agencies, this procedure may be delegated to trained nursing assistants or medical assistants. See Figure 19.1[8] for an image of a standard bedside glucometer kit that contains a glucometer, lancets, reagent strips, and calibration drops. Prior to performing a blood glucose test, read the manufacturer’s instructions and agency policy because they may vary across devices and sites. Ensure the glucometer has been calibrated per agency policy.[9]
Before beginning the procedure, determine if there are any conditions present that could affect the reading. For example, is the patient fasting? Has the patient already begun eating? Is the patient demonstrating any symptoms of hypoglycemia or hyperglycemia? Keep your patient safe by applying your knowledge of diabetes, the medication being administered, and the uniqueness of the patient to make appropriate clinical judgments regarding the procedure and associated medication administration.[10]
See the “Checklist for Blood Glucose Monitoring” for details regarding the procedure. It is often important to keep the patient’s hand warm and in a dependent position to promote vasodilation and obtain a good blood sample. If necessary, warm compresses can be applied for 10 minutes prior to the procedure to promote vasodilation. Follow the manufacturer’s instructions to prepare the glucometer for measurement. After applying clean gloves, clean the patient's skin with an alcohol wipe for 30 seconds, allow the site to dry, and then puncture the skin using the lancet. See Figure 19.2[11] for an image of performing a skin puncture using a lancet.
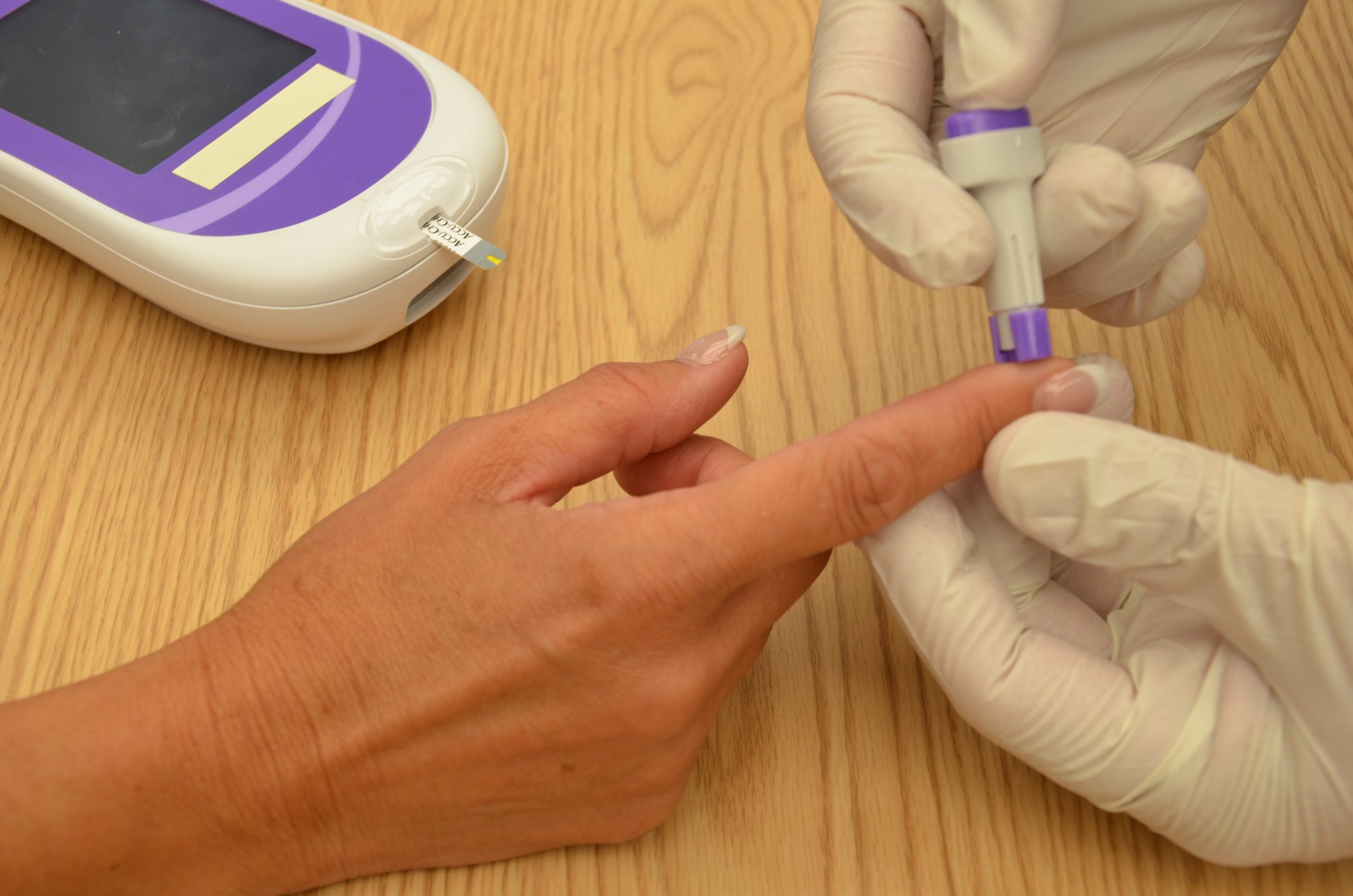
If needed, gently squeeze above the site to obtain a large drop of blood. Do not milk or massage the finger because it may introduce excess tissue fluid and hemolyze the specimen. Wipe away the first drop of blood and use the second drop for the blood sample. Follow agency policy and manufacturer instructions regarding placement of the drop of blood for absorption on the reagent strip. See Figure 19.3[12] for an image of a nurse absorbing the patient’s drop of blood on the reagent strip. Timeliness is essential in gathering an appropriate specimen before clotting occurs or the glucometer times out.

Cleanse the glucometer and document the blood glucose results according to agency policy. Report any concerns about patient symptoms or blood sugar results according to agency policy.
Life Span Considerations
Blood glucose samples should be taken from the heel of newborns and infants up to the age of six months. When obtaining a sample from the heel, the sample is taken from the medial or lateral plantar surface.
View a supplementary YouTube video on Obtaining a Bedside Blood Glucose[13]
The oropharynx is the part of the throat at the back of the mouth behind the oral cavity. It includes the back third of the tongue, the soft palate, the side and back walls of the throat, and the tonsils.[14] When obtaining a specimen from this area, it is important to avoid the tongue and teeth. Depending on the test ordered, the nurse may obtain the specimen from the tonsils alone or the posterior pharynx (throat) and the tonsils. Keep in mind that if you are ever unsure about how to accurately obtain a specimen, lab technicians are a great resource.
See the “Checklist for Oropharyngeal Testing” for additional details about performing the procedure.
Life Span Considerations
Infants and Children
Specimen collection on infants and children may require the support of another health care provider or a parent. Educate the patient and the parent about the procedure and the expectations if the parent decides to assist with the specimen collection. During specimen collection, it's important that the patient is immobile to prevent injury to the nasal cavity, nasopharyngeal, or oropharynx.
View a supplementary YouTube video from Medscape on How to Perform a Throat Swab[15].
Sputum specimens collected by expectoration are commonly used for cytology, culture and sensitivity, and acid-fast bacilli (AFB) testing. Cytologic examination identifies abnormal cells such as cancer. Culture and sensitivity testing identifies specific infectious microorganisms and their sensitivity to antibiotics. Optimally, sputum samples used for culture and sensitivity testing should be collected before initiating antibiotic therapy because antibiotics affect the results. AFB testing, along with culture and sensitivity testing, is used to diagnose tuberculosis (TB). When testing for TB, at least three consecutive samples are collected, with at least one being an early morning sample.
Prior to implementing the procedure, it is helpful to ensure the patient is well-hydrated. Hydration helps thin and loosen sputum and increases the likelihood of obtaining an adequate sample. If the patient is prescribed nebulizer treatments, it is helpful to administer this treatment prior to the procedure to help mobilize secretions. It is also important to assess if the patient is experiencing pain related to coughing. For example, pain following chest or abdominal surgery can inhibit the patient from taking deep breaths and expectorating. In this case, pain medication should be provided prior to performing the procedure. Patients can also be encouraged to support surgical wounds with a pillow while coughing to provide additional support and comfort.
It is best to obtain sputum samples in the early morning because secretions accumulate overnight. The patient can rinse their mouth with water prior to the procedure, but avoid mouthwash or toothpaste because these products can affect the microorganisms in the sample. Remove dentures if they are present.
Be aware that droplets and aerosols may be generated when collecting sputum specimens, so use appropriate personal protective equipment when entering the room and during the procedure based on the patient’s condition. Explain the procedure to the patient, the type of specimen required, and the difference between oral secretions and sputum. Position the patient in a seated position in a chair or at the side of the bed or place them in high Fowler’s position.
Instruct the patient to take three slow, deep breaths and then cough deeply. Repeat this process until the patient has produced sputum, with rest periods between each maneuver.
When the patient has mobilized sputum, instruct them to expectorate directly into a sterile specimen container without touching the inside or rim of the container. The specimen should be at least 5 mL (one teaspoon); ask the patient to continue producing and expectorating sputum until this amount is achieved. Assess the sputum specimen to ensure it is sputum and not saliva. Sputum appears thick and opaque, whereas saliva appears thin, clear, and watery.
Cap the specimen container tightly and ensure it is labeled with the patient's name. Place the specimen in a transport bag and send it to the laboratory for analysis. Document the time and date the sputum specimen was collected and the characteristics of the sputum, including amount and color.
If a patient is unable to expectorate a sputum sample, other interventions may be required to mobilize secretions. It is often helpful to collaborate with a respiratory therapist for assistance in this situation. Interventions may include nebulizers, hydration, deep-breathing exercises, chest percussion, and postural drainage. If these interventions are not successful, a sputum sample may be obtained via oropharyngeal or endotracheal suctioning.[16],[17]
Stool samples are collected from patients to test for cancer, parasites, or for occult blood (i.e., hidden blood). Follow specific instructions from the laboratory for collecting the sample.
The Guaiac-Based Fecal Occult Blood Test (gFOBT) is a commonly used test to find hidden blood in the stool that is not visibly apparent. As a screening test for colon cancer, it is typically obtained by the patient in their home using samples from three different bowel movements. Nurses may assist in gFOBT specimen collection during inpatient care.
Before the test, the patient should avoid red meat for three days and should not take aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen, for seven days prior to the test. (Blood from the meat can cause a false positive test, and aspirin and NSAIDS can cause bleeding, leading to a false positive result.) Vitamin C (more than 250 mg a day) from supplements, citrus fruits, or citrus juices should be avoided for 3 to 7 days before testing because it can affect the chemicals in the test and make the result negative, even if blood is present. If possible, avoid testing for occult blood during a female's menstrual cycle. The blood may contaminate the sample and cause a false positive.
To perform a gFOBT in an inpatient setting, perform the following steps.
- Verify the patient has not consumed red meat for three days, has not taken aspirin or NSAIDs for seven days prior to the test, and has not had vitamin C greater than 250 mg daily for the past 3-7 days because these substances can affect the results.
- Explain the procedure to the patient. Ensure collection container is placed within the toilet to collect specimen. Instruct them to flush the toilet before defecating to remove any potential chemicals and to not place toilet paper in the toilet after defecating. Request they notify you when they have had a bowel movement.
- Review the manufacturer's instructions because different test kits may have different instructions. Contact the laboratory with any questions.
- Label the card with the patient’s name and medical information per agency policy. Open the flap of the guaiac test card.
- Apply nonsterile gloves. Use the applicator stick to apply a thin smear of the stool specimen to one of the squares of filter paper on the card. Obtain a second specimen from a different part of the stool and apply it to the second square of filter paper on the card. (Occult blood isn’t typically equally dispersed throughout the stool.)
- Place the labeled test card in a transport bag and send it to the laboratory for analysis.
- If you are working in an agency where nurses apply the guaiac developer solution to the card, allow the specimen to dry for 3 to 5 minutes. Open the reverse side of the card and apply two drops of guaiac developer solution to each square. A blue reaction will occur within 60 seconds if the test is positive. The absence of a blue color after 60 seconds is considered a negative test.
- Document the date and time of the test and any unusual characteristics of the stool sample.[18],[19]
Sample Documentation of Expected Findings
Patient alert and oriented x 3, sitting in a wheelchair and awaiting breakfast. Patient denies symptoms of hypoglycemia or hyperglycemia. Bedside blood glucose obtained with results of 135 mg/dL. 2 units of regular insulin given subcutaneously left abdomen per sliding scale. Breakfast delivered to the patient.
Sample Documentation of Unexpected Findings
0730: Patient alert and oriented x 3, sitting in a wheelchair and awaiting breakfast. Denies symptoms of hypoglycemia or hyperglycemia. Bedside blood glucose obtained with results of 185 mg/dL. 6 units of regular insulin given per sliding scale along with 34 units of scheduled NPH insulin given subcutaneously left abdomen as breakfast tray was delivered to patient.
0900: Patient ate 25% of breakfast and complains of headache, fatigue, and dizziness. Patient is shaking and irritable but alert and oriented x 3. Blood glucose was rechecked and results were 65 mg/dL. 4 ounces of orange juice was provided.
0915: Blood glucose rechecked and results were 95 mg/dL. Patient states, “I’m feeling much better and not dizzy anymore.” Shakiness has resolved. Provided a peanut butter sandwich per patient request. Will continue to monitor the patient for signs of hypoglycemia. Call light within reach.

